Isobars and Isomers
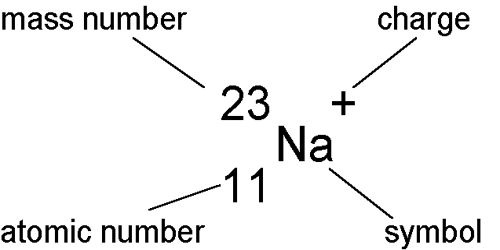
Isobars: Those atoms which have the same atomic weight or mass number but atomic numbers are different, they are called isobars. So, Atoms of different elements with different atomic numbers but have the same mass number are called isobars. Due to different atomic numbers, the isobars will have different atomic structures and therefore, will differ in chemical properties.
Example: 40Ar18 and 40Ca19 i.e., the mass number or atomic weight of both argon and calcium is 40 but their atomic numbers are respectively 18 and 19. So, they are isobars.
Isomers: Those atoms which have a same atomic number and atomic weight but internal structures are different, they are called isomers. Isomers are organic compounds which have the same molecular formula but different structural formula. Features of Isomers:
- They have a same molecular formula,
- They have a different structural formula,
- They vary in their chemical properties,
- They have different boiling and melting points.