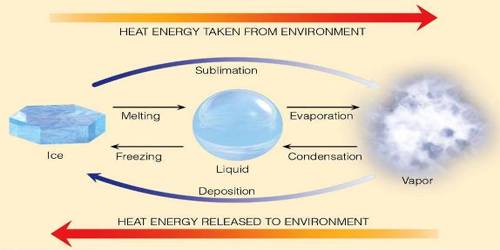
Latent Heat
It is the energy required to change a substance to a higher state of matter (solid > liquid > gas). This same energy is released from the substance when the change of state is reversed (gas > liquid > solid). It is the heat required to convert a solid into a liquid or vapor, or a liquid into a vapor, without change of temperature.
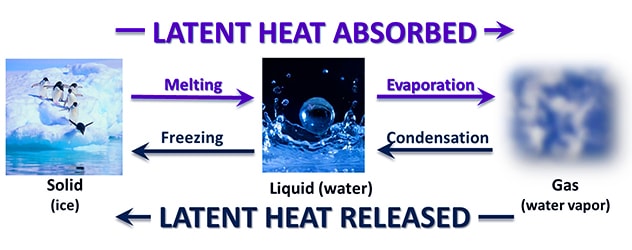
Latent heat can be understood as heat energy in a hidden form which is supplied or extracted to alter the state of a material without altering its temperature. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes, i.e. a material condensing or vaporizing at a particular temperature and pressure. It is the heat energy per mass unit essential for a phase alters to happen. If we think about substances at a molecular level, gaseous molecules have more vibration than liquid molecules. So when you add heat to a liquid, you are in fact causing the molecules to vibrate. The latent heat is the energy necessary to alter the molecular movement.
The term was introduced around 1762 by British chemist Joseph Black. It is derived from the Latin later (to lie hidden). Black used the term in the context of calorimetry where a heat transfer caused a volume change while the thermodynamic system’s temperature was constant.