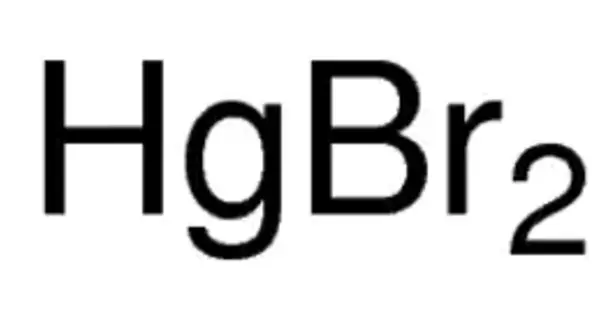The inorganic compound with the formula HgBr2 is mercury(II) bromide or mercuric bromide. It takes the form of white rhombic crystals. A laboratory reagent is this white solid. It is light-sensitive. It is slightly soluble in water and has a higher density than water. It is extremely toxic, as are all mercury salts. It is extremely toxic when inhaled or ingested.
Properties
Mercury dibromide is a mercury coordination entity with the formula HgBr2 that is made up of mercury and bromine.
- Chemical formula: HgBr2
- Molar mass: 360.41 g/mol
- Appearance: white solid
- Density: 6.03 g/cm3, solid
- Melting point: 237 °C (459 °F; 510 K)
- Boiling point: 322 °C (612 °F; 595 K)
- Solubility in water: 22 g/100 mL (25°C)
- Solubility product (Ksp): 6.2×10−20
- Solubility: 30 g/100 mL (25°C) ethanol
- Coordination geometry: rhombic
Preparation
Mercury(II) bromide can be created by reacting metallic mercury with bromine.
Mercury(II) bromide is a bromide of mercury that is primarily used as a laboratory reagent. Mercury is a heavy, silvery d-block metal and one of six elements that are liquid at or near room temperature and pressure. It is a naturally occurring substance that combines with other elements such as chlorine, sulfur, or oxygen to form inorganic mercury compounds (salts).
Reactions
The reagent mercury(II) bromide is used in the Koenigs-Knorr reaction, which forms glycoside linkages on carbohydrates. It is also used, as recommended by Pharmacopoeia, to test for the presence of arsenic. The arsenic in the sample is first converted to arsine gas by hydrogen treatment. Arsine reacts with mercury(II) bromide as follows:
AsH3 + 3HgBr2 → As(HgBr)3 + 3HBr
The white mercury(II) bromide will turn yellow, brown, or black if arsenic is present in the sample.
Mercury(II) bromide reacts violently with elemental indium at high temperatures and, when exposed to potassium, can form shock-sensitive explosive mixtures.
Uses
The reagent mercury(II) bromide is used in the Koenigs-Knorr reaction, which forms glycoside linkages on carbohydrates. It is also used, as recommended by the Pharmacopoeia, to test for the presence of arsenic.
Health Risk
TOXIC; inhaling, ingesting, or coming into contact with the material may result in severe injury or death. Contact with molten material can result in severe burns to the skin and eyes. Any skin contact should be avoided. Contact or inhalation effects may be delayed. Fire can emit irritant, corrosive, and/or toxic gases. Firefighting or dilution water runoff can be corrosive and/or toxic, resulting in pollution.