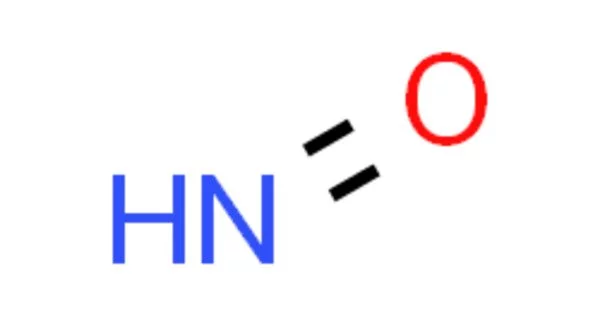
HNO is the chemical compound nitroxyl (common name) or azanone (IUPAC name). In the gas phase, it is well known. It is a molecule made up of one nitrogen (N) and one oxygen (O) atom, with a hydrogen (H) atom linked to the nitrogen atom. It is also referred to as “hyponitrous acid.”
Nitroxyl is a chemical molecule with a relatively brief life cycle that has piqued the curiosity of scientists and physicians due to its possible biological and physiological consequences. In the solution phase, it might develop as a short-lived intermediate. NO−, nitroxide anion, is the reduced form of nitric oxide (NO) that is isoelectronic with dioxygen. The bond dissociation energy of H−NO is 49.5 kcal/mol (207 kJ/mol), which is unusually weak for a bond to the hydrogen atom.
Properties
- Chemical formula: HNO
- Molar mass: 31.014 g·mol−1
- log P: 0.74
- Coordination geometry: Digonal
- Molecular shape: Bent
Nitroxyl has antioxidant properties and can scavenge harmful reactive oxygen species (ROS) in the body. This makes it potentially important for protecting cells from oxidative stress. It is highly reactive and has a very short half-life in biological systems. Its rapid conversion to other nitrogen oxide species, such as nitric oxide (NO) or nitrogen dioxide (NO2), complicates its study and therapeutic applications.
Detection
In biological samples, nitroxyl can be detected using fluorescent sensors, many of which are based on the reduction of copper(II) to copper(I) with concomitant increase in fluorescence. It is an isomer of nitric oxide (NO), another important signaling molecule in the body. It differs from NO by having one less electron, which leads to differences in its chemical behavior.
Medicinal chemistry
Nitroxyl donors, known as nitroso compounds, show potential in the treatment of heart failure and ongoing research is focused on finding new molecules for this task.
Biological Significance
Nitroxyl has been studied for its potential role in various biological processes. It has been proposed as an important signaling molecule in the cardiovascular system, with potential vasodilatory and cardioprotective effects.
Research and Medical Applications
Researchers are exploring the therapeutic potential of nitroxyl and related compounds in various medical conditions, including heart disease, hypertension, and neurodegenerative disorders. However, due to its reactivity and short half-life, developing stable and effective nitroxyl-based drugs is challenging.