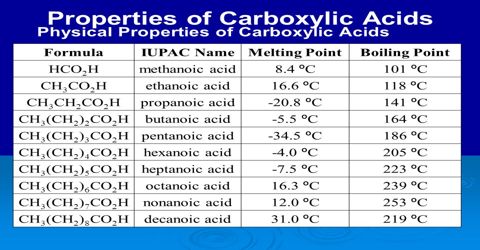
Physical properties of Acid Derivatives:
1. Acid chloride, Ester & Acid Anhydride:
- Volatileness: Acid chloride, ester & acid anhydrides can’t form H-bond by themselves. So they are volatile & have a characteristic odour. There is a dipole-dipole attraction force in each molecule of them. That’s why their lowest member is liquid in nature at room temp.
- Acid chlorides are liquid, have an irritating smell. Their vapours are tear-producers.
- Acid anhydrides are liquid, have an irritating smell. Aromatic anhydrides are solid.
- Esters are colorless liquid: have a sweet smell like fruits.
2. Acid amide: They can form H-bond. So they are solid in nature. Their boiling point is higher than that of corresponding carboxylic acids. They are comparatively more water soluble than other acid derivatives.
Chemical reactivity of aliphatic acid chlorides is more than that of aromatic acid chlorides. Mainly acetyl chloride. (CH3OCI) & acid anhydride, (CH3CO)2O are used as Acylating.
Reagent
- Chemical reactivity of acid derivatives RCOX > ROCOOCR’ > RCOOR’ > RCONH2
- Reactivity of organic acid inmate with the increasing number of Cl atoms in that acid. On the other hand, reactivity decreases with the increasing number of the methyl group.