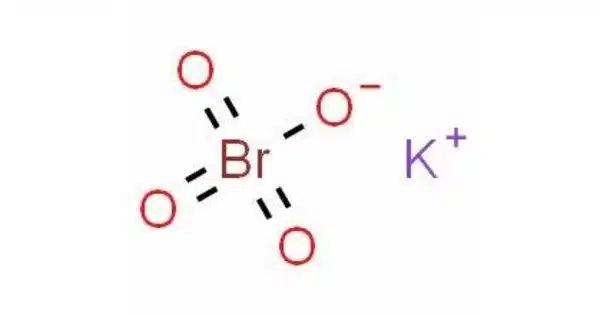
Potassium perbromate is the chemical compound composed of the potassium ion and the perbromate ion, with the chemical formula KBrO4. It is a chemical compound that contains potassium, bromine, and oxygen. It is a powerful oxidizing agent and is not commonly encountered in everyday life. Perbromates, including potassium perbromate, are typically used in laboratory and industrial settings for various chemical processes. It is a powerful oxidizing agent and is used in various chemical applications.
Properties
Potassium perbromate is typically a white crystalline solid, but its exact appearance can vary depending on its purity and form. is a strong oxidizing agent, meaning it has the ability to transfer oxygen atoms to other substances, causing them to undergo oxidation reactions. It is a stable compound when stored properly, but it should be kept away from reducing agents and combustible materials to prevent accidents.
- Chemical formula: KBrO4
- Molar mass: 183 g/mol
- Density: 3.08 g/cm3
- Melting Point: It decomposes before melting under normal atmospheric pressure.
- Boiling Point: It does not have a well-defined boiling point because it decomposes before reaching its boiling point.
Preparation
Potassium perbromate can be prepared by reacting perbromic acid with potassium hydroxide:
HBrO4 + KOH → KBrO4 + H2O
Application
Potassium perbromate is primarily used as an oxidizing agent in various chemical processes, such as in the production of pharmaceuticals and certain organic compounds. It is often used in chemical synthesis and analytical chemistry as an oxidizing agent. It can participate in various reactions where the transfer of oxygen atoms or oxidation of other substances is required. It can be used as an analytical reagent in laboratories. In some cases, it has been used as a disinfectant.
However, due to its powerful oxidizing properties, it should be handled with care, as it can be hazardous if not used properly.
Safety Precautions
Potassium perbromate can be hazardous if mishandled, as it can react vigorously with flammable and reducing substances. Proper safety measures and protective equipment should be used when working with this compound. It is important to handle it in a well-ventilated area or under a fume hood to avoid inhaling its dust or vapors.