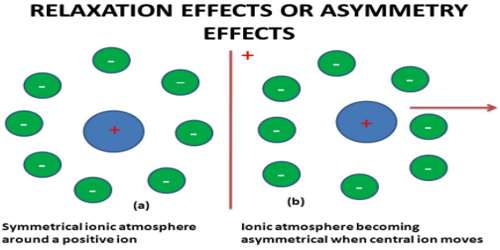
Relaxation effect is called the asymmetry effect, may be looked upon from a different perspective. Once the external field is applied, the central ion starts moving toward the electrode of opposite charge and more of the ionic atmosphere is left behind that is present on the front side. These excess ions of the ionic atmosphere which are left behind will tend to retard the speed of the moving ion due to the electrostatic force. Thus the effect arises because of the asymmetry of the ionic atmosphere, and hence the effect is called asymmetry effect. An approximate representation of the above statements is given in Figure.
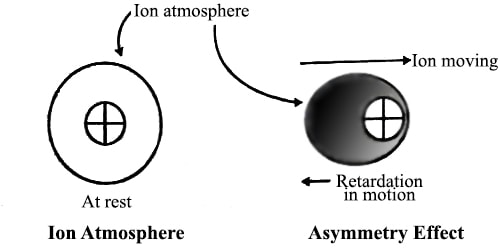
The arrow at the top of the ‘ion atmosphere’ represents the velocity with which the cation would have moved to the cathode if no retarding force was in operation. The small arrow below the ion atmosphere shows the retarding force. Since in a solution of one mole of the electrolyte the number of tons is constant this retardation of the velocity of the ions brings about an overall decrease in molar conductance from its ideal value that the solution would have in absence of any other force.