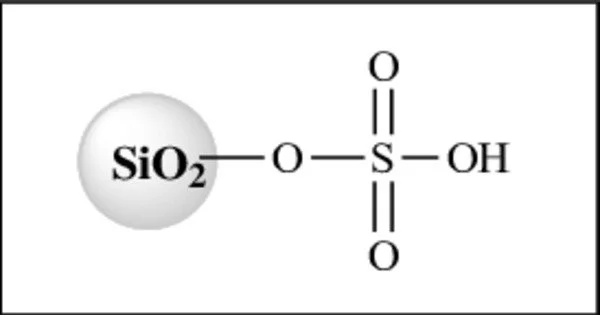
Silica sulfuric acid, also known as silicon dioxide sulfuric acid, is a type of acid catalyst commonly used in organic chemistry reactions. It is prepared by adding concentrated sulfuric acid to a mixture of silica gel and water, resulting in the formation of a viscous, clear liquid.
It is prepared by soaking silica gel with sulfuric acid. SSA is used as a catalyst in different organic synthesis process. Silica sulfuric acid is considered as cheap, nonhazardous, and easy-to-handle solid acid catalyst with high acidity.
Properties
- Appearance: SSA is a viscous, clear, and colorless liquid.
- Acidic nature: SSA is a very strong acid with a low pH. It is highly corrosive and can cause severe burns if it comes into contact with skin or eyes.
- Solubility: SSA is soluble in polar solvents such as water, methanol, and ethanol, but insoluble in nonpolar solvents such as hexane.
- Stability: SSA is stable under normal conditions but can decompose upon exposure to high temperatures or water.
- Reactivity: SSA is a powerful catalyst for various organic reactions such as esterification, acylation, and alkylation.
- Storage: SSA should be stored in a cool, dry, and well-ventilated area away from heat, sources of ignition, and incompatible materials.
Preparation
It is prepared by adding concentrated sulfuric acid to silica gel, which leads to the formation of a solid, porous material that contains a high concentration of acid sites. Silica sulfuric acid is often used in reactions involving alcohols, ethers, and esters, as well as in dehydration and condensation reactions.
One of the advantages of silica sulfuric acid is that it can be easily handled and stored, and it is less corrosive than many other strong acids such as hydrochloric or sulfuric acid. It is also reusable and can be regenerated by heating it to high temperatures to remove the adsorbed water and organic compounds.
Synthesis
In a typical process, SSA is prepared by soaking silica gel into sulfuric acid of an appropriate concentration. Then moisture and water content of the mixture was evaporated by heating at high temperature (120–180°C).
SiO2–H2O + H2SO4 → SiO2–H2SO4 + H2O
Application
Silica sulfuric acid is mostly used as a solid acid catalyst in organic conversion. This acid catalyst is commonly used in a variety of reactions, including esterification, hydration of olefins, and the Friedel-Crafts reaction. It is known for its ability to promote reactions that are normally slow or unfeasible under normal conditions.
However, silica sulfuric acid should be handled with care as it is still a strong acid and can cause severe burns if it comes into contact with the skin or eyes. It should be used in a well-ventilated area to avoid inhaling the fumes, and proper personal protective equipment, such as gloves and safety goggles, should be worn.