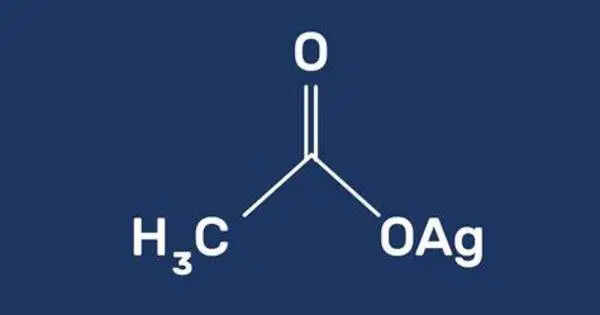
Silver acetate is a coordination compound with the empirical formula CH3CO2Ag (or AgC2H3O2). A photosensitive, white, crystalline solid, it is a useful reagent in the laboratory as a source of silver ions lacking an oxidizing anion. It has been used in organic synthesis as a mild oxidizing agent. It can be employed in reactions to introduce silver ions into organic compounds.
Properties
It is generally a white or light-sensitive crystalline solid. It is sparingly soluble in water. Its solubility can be influenced by factors such as temperature and the presence of other substances. It is relatively stable under normal conditions but may decompose upon heating.
- Chemical formula: AgC2H3O2
- Molar mass: 166.912 g/mol
- Appearance: white to slightly grayish powder
- Slightly: acidic odor
- Density: 3.26 g/cm3, solid
- Melting point: 220 °C (428 °F; 493 K) (decomposes)
- Solubility in water: 1.02 g/100 mL(20 °C)
- Solubility product (Ksp): 1.94×10−3
Reactions
Silver acetate finds use in certain transformations in organic synthesis.
(a) Sulfenamide synthesis
Silver acetate is used to prepare sulfenamides from disulfides and secondary amines:[5]
R2NH + AgOAc + (RS)2 → R2NSR + AgSR + HOAc
(b) Hydrogenation
A solution of silver acetate in pyridine absorbs hydrogen, producing metallic silver:[6]
2 CH3CO2Ag + H2 → 2 Ag + 2 CH3CO2H
(c) Direct ortho-arylation
It is a reagent for direct ortho-arylation (to install two adjacent substituents on an aromatic ring) of benzylamines and N-methylbenzylamines. The reaction is palladium-catalyzed and requires a slight excess of silver acetate.[7] This reaction is shorter than previous ortho-arylation methods.
Applications
While it is not as commonly used as some other silver compounds, silver acetate has found applications in certain chemical reactions and laboratory procedures. Its use may depend on the specific requirements of a given synthesis or process.
Toxicity
Like many silver compounds, silver acetate can be toxic, and precautions should be taken when handling it. Contact with skin and eyes should be avoided, and inhalation of its dust or vapors should be minimized.