Transition State Theory
It is now believed that all chemical processes proceed through the formation of an intermediate activated complex or transition state. The activated complex is a grouping of atoms of reactant molecules which is unstable. The activated complex breaks up or decomposes at a definite rate to give the products of the reaction. Consider a reaction of the type:
AB + C → AC + B
It is thought that at first an activated complex, having a structure intermediate between that of the reactants and products, is formed. In the above example the reaction may be written as:
AB + C → A…B…C → AC (activated complex) + B
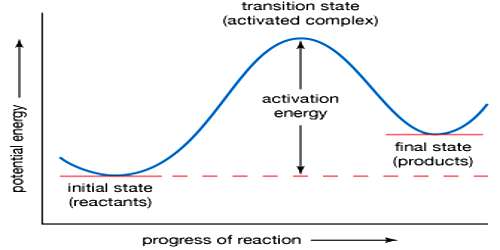
The activated complex (A…B…C) has a higher potential energy than either the reactants or products. The change of potential energy with ‘progress’ of the reaction, i.e., the reaction co-ordinate is diagrammatically represented in Figure.
The difference in energy between AB + C and the activated complex is equal to Ea, the energy of activation of the reaction, as shown in equation. It is to be noted that to get from the reactant side to the product side the system has to cross over an energy barrier corresponding to the energy of the activated complex. In spite of collision between molecules reaction cannot take place unless the energy barrier can be crossed. The difference between the energy of the reactants and that of the products is the enthalpy change of the reaction (ΔH). As the energy level of the products is lower than that of the reactants, energy is given out during the reaction, the reaction being exothermic. In endothermic reactions the energy level of products is between that of the reactants and the activated complex.
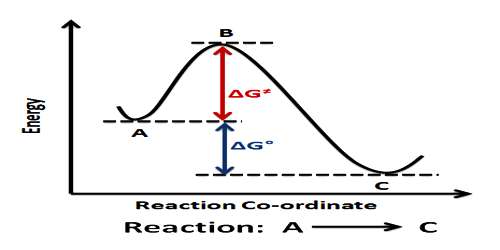
Figure: Potential Energy at a function of reaction coordinates
A proper understanding of the activated complex needs carefully study and imagination. In the case of the reaction between hydrogen and iodine to form hydrogen iodide the activated complex has been represented as below:
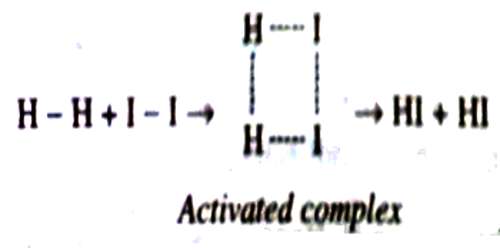
As the reactant molecules become more complicated the activated complex becomes more difficult to visualize.