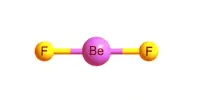
Liquid Crystals that exhibit a phase of matter that has properties between those of a conventional liquid and those of a solid crystal. For instance, a liquid crystal (LC) may flow like a liquid, but have the molecules in the liquid arranged and or oriented in a crystal-like way. These are the substances having a state of aggregation intermediate to highly ordered solid and disordered liquid. That is intermediate state between solid and isotropic liquid. These intermediate phases are called “Liquid Crystal Phase or Mesophase”; meso means in between (the crystal and the liquid phase).Liquid crystals may also be called crystalline liquids.
In 1888, the Austrian botanical physiologist Friedrich Reinitzer (1858-1927), (interested in the biological function of cholesterol in plants) was looking at the melting behavior of an organic substance related to cholesterol (cholesteryl benzoate and cholesteryl acetate).
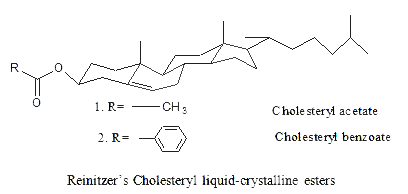
He found out that cholesterol benzoate does not melt like other compounds but obviously had two melting points. At 145.5°C, it melted into a cloudy liquid and at 178.5°C, it melted again and cloudy liquid suddenly became clear. Furthermore, the phenomenon was reversible. Discussion with Otto Lehmann and others led to the identification of a new phase of matter called the liquid crystal phase.