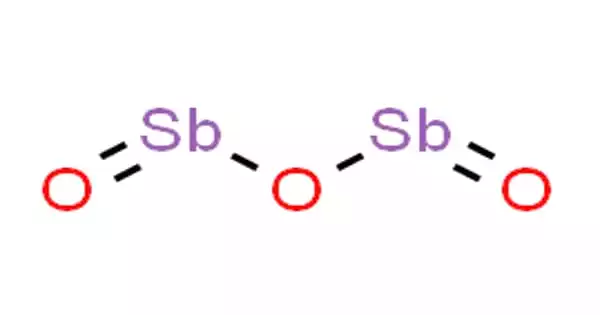
Antimony(III) oxide is an inorganic chemical with the formula Sb2O3. It is the most major antimony commercial chemical. It can be found in nature as the minerals valentinite and senarmontite. Sb2O3, like other polymeric oxides, dissolves in aqueous solutions by hydrolysis. The extremely rare mineral stibioclaudetite contains a mixed arsenic-antimony oxide.
Properties
Antimony(III) oxide is an amphoteric oxide. It dissolves in aqueous sodium hydroxide solution to form the meta-antimonite NaSbO2, which can be separated as the trihydrate. Antimony(III) oxide dissolves in concentrated mineral acids to form the appropriate salts, which hydrolyze when diluted with water. The trioxide is converted to antimony(V) oxide using nitric acid.
- Chemical formula: Sb2O3
- Molar mass: 291.518 g/mol
- Appearance: white solid
- Odor: odorless
- Density: 5.2 g/cm3, α-form; 5.67 g/cm3 β-form
- Melting point: 656 °C (1,213 °F; 929 K)
- Boiling point: 1,425 °C (2,597 °F; 1,698 K) (sublimes)
- Solubility in water: 370 ± 37 µg/L between 20.8°C and 22.9°C
- Solubility: soluble in acid
When heated with carbon, the oxide is reduced to antimony metal. With other reducing agents such as sodium borohydride or lithium aluminium hydride, the unstable and very toxic gas stibine is produced. When heated with potassium bitartrate, a complex salt potassium antimony tartrate, KSb(OH)2•C4H2O6, is formed.

Production
Global production of antimony(III) oxide in 2012 was 130,000 tonnes, an increase from 112,600 tonnes in 2002. China produces the largest share followed by US/Mexico, Europe, Japan and South Africa and other countries (2%).
Antimony(III) oxide was manufactured at four sites in the EU27 as of 2010. It is manufactured in two ways: by re-volatilizing crude antimony(III) oxide and by oxidizing antimony metal. In Europe, antimony metal oxidation reigns supreme. There are several ways for producing crude antimony(III) oxide or metallic antimony from virgin material. The technique chosen is determined on the nature of the ore and other factors. Mining, crushing, and grinding of ore are common phases, followed by froth flotation and metal separation using pyrometallurgical processes (smelting or roasting) or, in some situations (for example, when the ore is rich in precious metals), hydrometallurgical operations. These steps take place outside of the EU, closer to the mining site.
Structure
The temperature of the sample influences the structure of Sb2O3. The high temperature (1560 °C) gas is dimeric Sb4O6. Sb4O6 molecules are bicyclic cages, comparable to phosphorus trioxide, a related oxide of phosphorus(III). The cage structure is preserved in a solid that crystallizes cubically. The O-Sb-O angle is 95.6° and the Sb-O distance is 197.7 pm. In nature, this form exists as the mineral senarmontite. Above 606 °C, the more stable form is orthorhombic, with pairs of -Sb-O-Sb-O- chains connected by oxide bridges between the Sb centers. In nature, this form exists as the mineral valentinite.
Uses
In the United States and Europe, the yearly consumption of antimony(III) oxide is around 10,000 and 25,000 tonnes, respectively. The primary application is as a synergist flame retardant in conjunction with halogenated compounds. The mix of halides and antimony is critical to polymer flame retardancy, assisting in the formation of less flammable chars. These flame retardants can be found in electrical equipment, fabrics, leather, and coatings.
Other applications:
- It is an opacifying agent for glasses, ceramics and enamels.
- Some specialty pigments contain antimony.
- It is a useful catalyst in the production of polyethylene terephthalate (PET plastic) and the vulcanization of rubber.
Safety
Antimony(III) oxide is thought to be carcinogenic to humans. As with other antimony compounds, its TLV is 0.5 mg/m3. There are no other human health dangers associated with antimony(III) oxide, and there are no risks to human health or the environment associated with the manufacturing and use of antimony trioxide in everyday life.