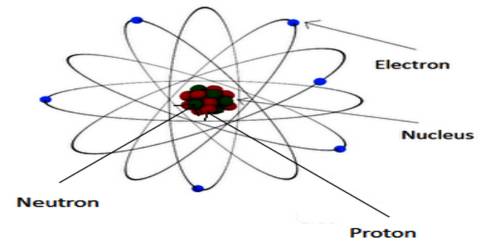
The atomic concept of magnetism: We know, a material is composed of atoms and molecules. There are protons and neutrons in the nucleus of an atom and electrons revolve around the nucleus in different orbits. Again, electrons have rotation and spin motion about their own axes. The moments associated with the orbital motion and spin motion are called orbital motion moment and spin motion moment, respectively. The magnetic moment associated with the nucleus of the atom is called nuclear magnetic moment.
The atomic theory of magnetism was given by Weber and modified by Ewing. According to this theory:
- Each and every molecule of a magnetic substance is a complete magnet in itself, having a north pole and a south pole of equal strength.
- When the substance is magnetized, the molecular magnets are realigned so that north poles of all molecular magnets point in one direction and south poles of all molecular magnets point in the opposite direction.
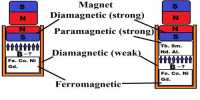
Due to the resultant action of these moments different magnetic characters and properties are manifested. Depending on the behaviour materials are classified into paramagnetic, diamagnetic, and ferromagnetic materials. Using powerful magnets Faraday examined and observed that some materials are attracted by the magnets and some are repelled by the magnates. Materials which are attracted by the magnet are called paramagnetic materials. As for example irons nickel, aluminium, cobalt etc. On the other hand, the materials which are repelled by the magnet are called diamagnetic materials. As for example, bismuth, antimony, zinc etc. Again, among paramagnetic materials, some are attracted strongly. There are called ferromagnetic materials. As for example soft iron, steel etc.