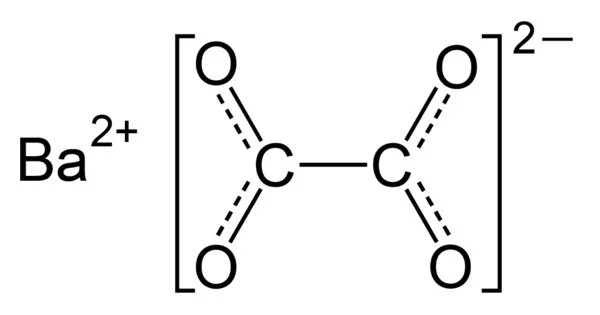
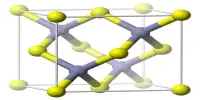
Barium oxalate (BaC2O4) is a white odorless powder that is occasionally used as a green pyrotechnic colorant in specialized pyrotechnic compositions containing magnesium metal powder. It is a white, odorless powder that is used as a green pyrotechnic colorant in specialized pyrotechnic compositions that contain magnesium.
Without any additional chlorine donors, the flame color is rich and vivid. The burn rate of such compositions is met without the use of commonly used oxidizers such as nitrates, chlorates, and perchlorates. It is a reducing agent rather than an oxidizing agent, which distinguishes it from most pyrotechnic colorants.
Properties
Despite being relatively stable, barium oxalate can be reactive with strong acids. The substance, a mild skin irritant, is toxic when ingested, causing nausea, vomiting, kidney failure, and gastrointestinal tract injury. It is a reducing agent rather than an oxidizing agent, which distinguishes it from most pyrotechnic colorants. It is extremely insoluble in water and, when heated, converts to the oxide form.

- Chemical formula: BaC2O4
- Molar mass: 225.346 g/mol
- Density: 2.658 g/cm3
- Melting point: 400 °C (752 °F; 673 K) (decomposes)
- Solubility in water: 0.9290 mg/L
Preparation
The raw materials that are required to prepare barium oxalate are oxalic acid and barium hydroxide (or its octahydrate).
It can also be prepared by using an oxalic acid solution and a barium chloride solution, with the reaction as follows:
BaCl2 + H2C2O4 → BaC2O4↓ + 2 HCl
White barium oxalate is formed when soluble oxalates react with barium ion. Strong acids and hot dilute acetic acid dissolve this precipitate.
The flame test: A Bunsen burner flame is colored yellow-green by barium salt solutions.
It is a white, odorless powder that is occasionally used as a green pyrotechnic colorant in specialized pyrotechnic compositions that contain magnesium. While it is generally stable, it can be reactive with strong acids. The substance, a mild skin irritant, is toxic when ingested, causing nausea, vomiting, and renal failure.