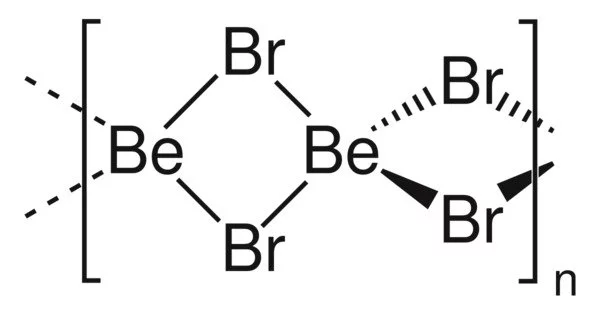
Beryllium bromide, also known as BeBr2, is a chemical compound with the formula BeBr2. It is highly hygroscopic and readily dissolves in water. It is a polymer with tetrahedral Be centers.
Beryllium bromide is a beryllium bromide. Beryllium is an atomic number 4 lightweight alkaline earth metal. It is a relatively uncommon element that occurs naturally only in combination with other elements in minerals. Bromine is a halogen element with the atomic number 35 and the symbol Br.
Properties
- Chemical formula: BeBr2
- Molar mass: 168.820 g/mol
- Appearance: colorless white crystals
- Density: 3.465 g/cm3 (20 °C)
- Melting point: 508 °C (946 °F; 781 K)sublimes at 473 °C (883 °F; 746 K)
- Boiling point: 520 °C (968 °F; 793 K)
- Solubility in water: Highly
- Solubility: soluble in ethanol, diethyl ether, pyridine, insoluble in benzene
- Crystal structure: Orthorhombic
Preparation and reactions
It can be prepared by reacting beryllium metal with elemental bromine at temperatures of 500 °C to 700 °C:
Be + Br2 → BeBr2
Beryllium bromide is also formed when treating beryllium oxide with hydrobromic acid:
BeO + 2 HBr → BeBr2 + H2O
It hydrolyzes slowly in water: BeBr2 + 2 H2O → 2 HBr + Be(OH)2
Structure
BeBr2 exists in two forms (polymorphs). Both structures are made up of tetrahedral Be2+ centers linked together by doubly bridging bromide ligands. Edge-sharing polytetrahedra are one form. The other form has interconnected adamantane-like cages that resemble zinc iodide.
Preparation
BeBr2 is the chemical formula for beryllium bromide. It is highly hygroscopic and water soluble. It has a molar mass of 168.821 g/mol. There is no documentation for the structure. Beryllium bromide is made by reacting Be metal with elemental bromine at temperatures ranging from 500°C to 700°C:
Be+Br2→BeBr2
Beryllium bromide is also formed when beryllium oxide with HBr in aqueous solution or hydrogen bromide in the gas phase:
BeO +2HBr →BeBr2 + H2O
Wohler (1828) created this bromide compound by reacting bromine vapor with metal and a mixture of carbon and beryllium oxide. By dissolving the oxide in hydrobromic acid, Berthemot (1831) obtained it in solution. Humpridge (1883) also made it by reacting the oxide and carbon with dry bromine.
Sublimation always yields colorless white crystals of anhydrous bromide.
Applications
Beryllium fluoride is used in biochemistry, particularly protein crystallography, because it binds in human tissues in some of the same ways that phosphate does. ADP and beryllium fluoride tend to bind to ATP sites and inhibit protein action when combined.
Safety
Beryllium compounds are toxic if inhaled or ingested.