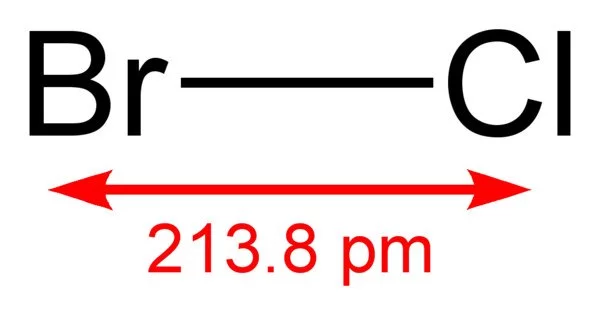
Bromine monochloride is an interhalogen inorganic compound with the chemical formula BrCl. It is also known as bromine(I) chloride, bromochloride, and bromine chloride. It is a highly reactive golden yellow gas with a boiling point of 5 degrees Celsius and a melting point of 66 degrees Celsius. It has the CAS number 13863-41-7 and the EINECS number 237-601-4. It is a powerful oxidizing agent.
Bromine chloride is a reddish-yellow mobile liquid with an unpleasant odor. Toxic when ingested or inhaled, and irritating to the skin, eyes, and mucous membranes. Prolonged exposure to high temperatures may cause the containers to explode violently. As an industrial disinfectant, it is used.
Properties
- Chemical formula: BrCl
- Molar mass: 115.357 g/mol
- Density: 2.172 g/cm3
- Melting point: −54 °C (−65 °F; 219 K)
- Boiling point: 5 °C (41 °F; 278 K)
- Solubility in water: 8.5 g/L

Uses
Bromine monochloride is used in analytical chemistry to quantitatively oxidize mercury in the sample to Hg(II) state in order to determine low levels of mercury. Bromine monochloride is commonly used as an algaecide, fungicide, and disinfectant in industrial recirculating cooling water systems. Bromine monochloride is added to some Li-SO2 batteries to increase voltage and energy density.
It is used in the production of fire-retardant chemicals, pharmaceuticals, high-density brominated liquids, agricultural chemicals, dyes, and bleaching agents as a brominating agent.
Safety
It is a toxic reddish-yellow mobile liquid or gas that is highly oxidizing. The substance has corrosive effects on the eyes, skin, and respiratory tract at first, resulting in chronic inflammation and impaired functions. Asthma, pneumonitis, and lung edema may result from inhalation.