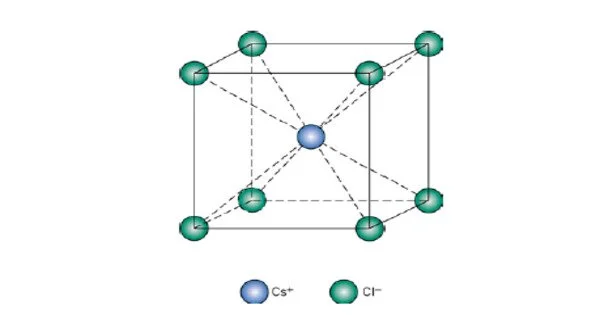
Caesium chloride, abbreviated CsCl, is an inorganic compound. It is the inorganic chloride salt of caesium, with eight chlorine ions coordinating each caesium ion. This colourless salt is a valuable source of caesium ions in a wide range of niche applications. Each caesium ion is coordinated by 8 chloride ions in its crystal structure, forming a major structural type.
In water, caesium chloride dissolves. It functions as a phase-transfer catalyst as well as a vasoconstrictor agent. It is a combination of an inorganic chloride and an inorganic caesium salt. When heated, CsCl transforms into NaCl. Caesium chloride occurs naturally as an impurity in carnallite, sylvite, and kainite (up to 0.002%). Globally, less than 20 tonnes of CsCl are produced each year, the majority of which comes from the caesium-bearing mineral pollucite.
Physical properties
Caesium chloride is colourless when crystallized and white when powdered. It dissolves easily in water, with maximum solubility ranging from 1865 g/L at 20 °C to 2705 g/L at 100 °C. The crystals are highly hygroscopic and gradually disintegrate at room temperature. Caesium chloride has no hydrates.
- Chemical formula: CsCl
- Molar mass: 168.36 g/mol
- Appearance: white solid, hygroscopic
- Density: 3.988 g/cm3
- Melting point: 646 °C (1,195 °F; 919 K)
- Boiling point: 1,297 °C (2,367 °F; 1,570 K)
- Solubility in water: 1910 g/L (25 °C)
- Solubility: soluble in ethanol

Application
Caesium chloride is a common medicine structure used in isopycnic centrifugation to separate different types of DNA. It is a reagent used in analytical chemistry to identify ions based on the colour and morphology of the precipitate.
Caesium chloride, when enriched in radioisotopes such as 137CsCl or 131CsCl, is used in nuclear medicine applications such as cancer treatment and myocardial infarction diagnosis. Another type of cancer treatment was investigated using non-radioactive CsCl. While conventional caesium chloride is relatively safe for humans and animals, the radioactive form easily contaminates the environment due to CsCl’s high solubility in water.
Toxicity
Caesium chloride is not toxic to humans or animals. In mice, the median lethal dose (LD50) for oral administration is 2300 mg per kilogram of body weight and 910 mg/kg for intravenous injection. CsCl’s low toxicity is due to its ability to lower potassium concentrations in the body and partially substitute it in biochemical processes. When taken in large amounts, however, it can cause a significant potassium imbalance, leading to hypokalemia, arrhythmia, and acute cardiac arrest. Caesium chloride powder, on the other hand, can irritate the mucous membranes and cause asthma.