Bonding in Molecular Crystals and their Characteristic
Depending on the forces that hold the atoms, molecules or ions together in crystal lattice crystals are classified into four main types.
These are: (i) Ionic crystal, (ii) Molecular crystal, (iii) Network covalent crystal and (iv) Metallic crystal. Here briefly focus on Molecular crystal.
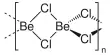
Fig: bonding pattern in N8 molecules
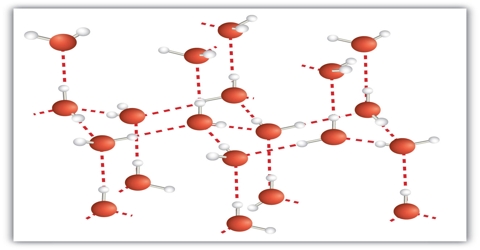
Molecular crystal: The lattice points in molecular crystals are occupied by molecules which are held together either by relatively weak van der Waal’s force or hydrogen bonding. As a result the molecular crystals are soft and have low melting points. Solid sulfur dioxide (SO2) is an example of molecular crystal. The predominant force in solid sulfur dioxide is dipole-dipole interaction. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Ice is another example of molecular crystal which has a three dimensional lattice structure. Intermolecular hydrogen bonding is responsible for maintaining this three dimensional network in ice. Other examples of molecular crystals are I2, P4, S8, CCl4 etc.