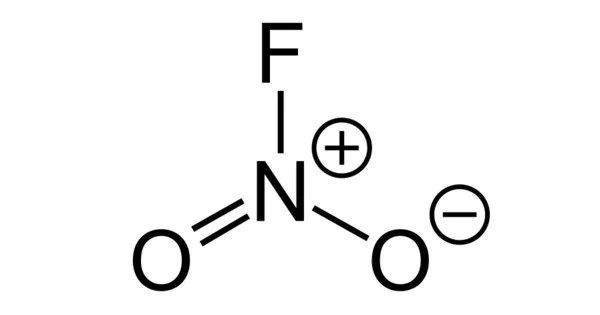
Nitryl fluoride, (NO2F) is a colorless gas and a strong oxidizing agent that is used as a fluorinating agent and has been proposed as an oxidizer in rocket propellants (though never flown).
The Nitryl fluoride molecule is made up of one or more Nitrogen atoms, two Oxygen atoms, and one Fluorine atom (s). The exact term for the above molecular weight is “molar mass,” which is based on each element’s atomic mass. The term “molecular weight” is an older term for “relative molar mass” or “molecular mass,” which is a dimensionless quantity equal to the molar mass divided by the molar mass constant defined as 1 g/mol. It is a molecular species, not an ionic species, as evidenced by its low boiling point. The structure features planar nitrogen with a short N-F bond length of 135 pm.
Properties
- Chemical formula: FNO2
- Molar mass: 65.003 g·mol−1
- Melting point: −166 °C (−267 °F; 107 K)
- Boiling point: −72 °C (−98 °F; 201 K)
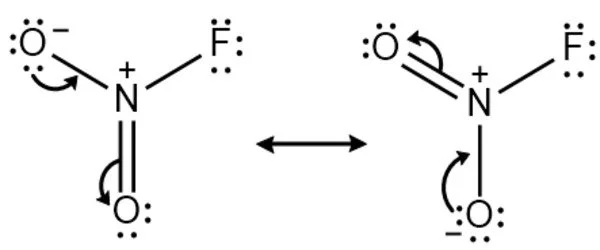
Preparation
In 1905, Henri Moissan and Paul Lebeau reported the fluorination of nitrogen dioxide to produce nitryl fluoride. This reaction is extremely exothermic, resulting in contaminated products. The most basic method uses cobalt(III) fluoride instead of fluorine gas:
NO2 + CoF3 → NO2F + CoF2
The CoF2 can be regenerated to CoF3. Other methods have been described.
Thermodynamic properties
- IR and Raman spectroscopy were used to determine the thermodynamic properties of this gas. FNO2 has a standard heat of formation of -19 ± 2 kcal/mol.
- At 500 kelvin, the equilibrium of the unimolecular decomposition of FNO2 is at least six orders of magnitude on the side of the reactants, and two orders of magnitude at 1000 kelvin.
- At temperatures below 1200 kelvin, the homogeneous thermal decomposition cannot be studied.
- With increasing temperature, the equilibrium shifts toward the reactants.
The dissociation energy of the N-F bond in nitryl fluoride is 46.0 kcal, which is approximately 18 kcal less than the normal N-F single bond energy. This is due to the NO2 radical’s “reorganization energy,” which means that the NO2 radical in FNO2 is less stable than the free NO2 molecule. In terms of quality, the odd electron “used up” in the N-F bond forms a resonating three-electron bond in free NO2, stabilizing the molecule with an 18 kcal gain.
Reactions
Organic nitro compounds and nitrate esters can be made using nitryl fluoride.