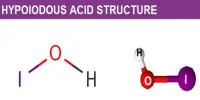
When a piece of iron is brought in contact with dilute sulphuric acid, a vigorous reaction begins and hydrogen gas starts coming out. The same piece of iron will displace copper from a solution of copper sulphate. It is noticed, however, that if the iron piece is kept for some time in conc. HNO3, and then washed free from nitric acid and allowed to react in the above manner, it can neither liberate hydrogen from H2SO4 nor precipitate copper from copper sulphate solution. In other words, by treatment with nitric acid iron has so changed that it would not behave in the same manner as any other untreated piece of iron would do. Iron in this form is said to be passive, i.e., it shows lesser reactivity than it would normally show.
Theory of Passivity :
Oxide Film Theory: Faraday suggested that the passivity of iron is due to the formation of an extremely thin and impervious film on the surface of iron. This film is usually of ferrosoferric oxide, Fe3O4.
2HNO3 (conc.) = 2NO2 + H2O + [O]
3Fe + 4[O] = Fe3O4
This theory is confirmed by the fact that if the oxide film on the surface is removed by scratching or by heating it in a reducing atmosphere of H2 or CO or by dissolving it in iodine solution, iron again becomes active and begins to give its usual reactions.
Fe3O4 + 4H2 = 3Fe + 4H2O