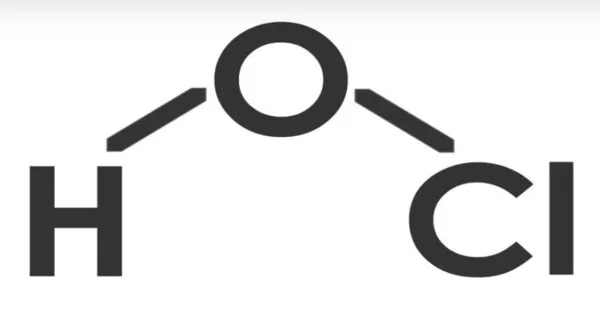
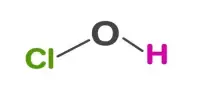
Hypochlorous acid (ClOH, HClO, HOCl, or ClHO) is a weak acid formed when chlorine dissolves in water and partially dissociates to form hypochlorite, ClO. HClO and ClO are oxidizers and the primary disinfectants in chlorine solutions. It is also known as chlorine oxide or chloric(I) acid. Because of its rapid equilibration with its precursor, chlorine, HClO cannot be isolated from these solutions.
The related compounds sodium hypochlorite (NaClO) and calcium hypochlorite (Ca(ClO)2) are used in many commercial bleaches, deodorants, and disinfectants due to their strong antimicrobial properties. Hypochlorous acid is also found in the white blood cells of mammals, including humans, as a defense against foreign bodies. HOCl is produced in living organisms by the reaction of hydrogen peroxide with chloride ions catalyzed by the heme enzyme myeloperoxidase (MPO).
Hypochlorous acid solutions, like many other disinfectants, will kill pathogens absorbed on surfaces, such as COVID-19. Such solutions, in low concentrations, can be used to disinfect open wounds.
Properties
- Chemical formula: HClO
- Molar mass: 52.46 g/mol
- Appearance: Colorless aqueous solution
- Density: Variable
- Solubility in water: Soluble
- Acidity (pKa): 7.53
- Conjugate base: Hypochlorite
Commercialization
Despite being discovered a long time ago, the stability of hypochlorous acid water for disinfection is difficult to maintain. The active compounds quickly deteriorate back into salt water in solution, losing their disinfecting capability and making it difficult to transport for widespread use. Despite its superior disinfectant properties, it is less commonly used as a disinfectant than bleach and alcohol due to its higher cost.
Technological advancements have reduced manufacturing costs and enabled the production and bottling of hypochlorous acid water for residential and commercial use. Hypochlorous acid has gained popularity in recent years as a safe and environmentally friendly alternative to traditional chemical disinfectants. When used in appropriate concentrations, it is considered safe for use around humans, animals, and plants. It also does not leave any harmful residues, making it suitable for use in healthcare, food processing, and household cleaning.
Applications
Because of its ability to kill bacteria, viruses, and other microorganisms, HOCl is an important oxidizing agent and disinfectant. Our immune system naturally produces it as part of the body’s defense mechanism against infections. When white blood cells detect pathogens such as bacteria or viruses, they release HOCl to neutralize and destroy them.
Hypochlorous acid is widely used for disinfection in a variety of industries. It is frequently used as a water treatment agent to purify drinking water and disinfect swimming pools. Its potent antimicrobial properties enable it to effectively eliminate a wide variety of harmful microorganisms.
Safety
It’s essential to handle hypochlorous acid with care, as it can still be hazardous in its concentrated form. Diluted solutions are recommended for most applications, and it’s essential to follow proper safety guidelines and instructions provided by manufacturers or experts when using this chemical.