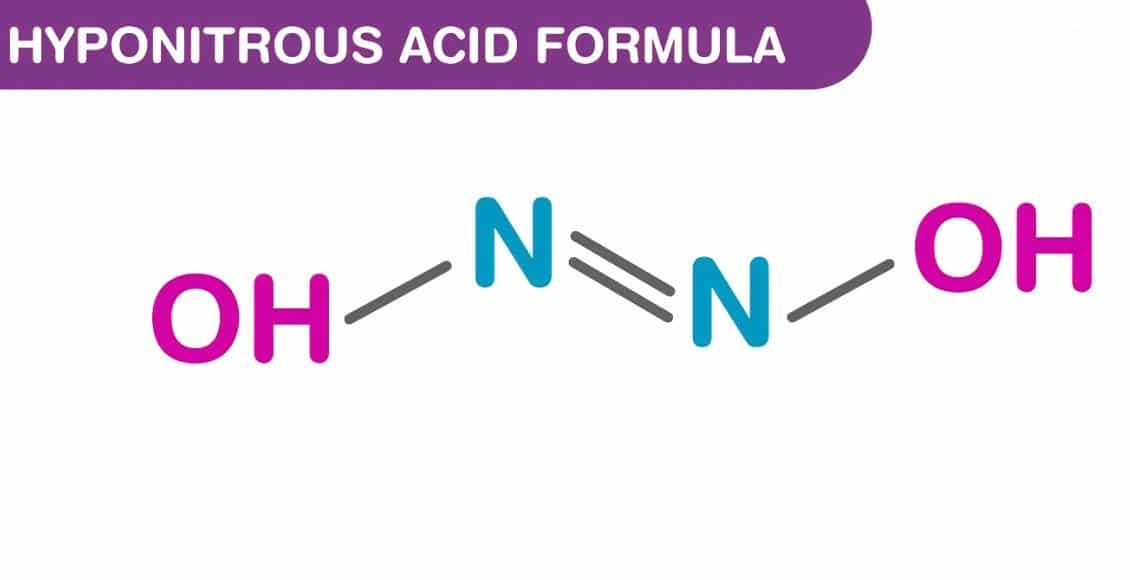
Hyponitrous acid is a chemical compound with formula H2N2O2 or HON=NOH. It is an isomer of nitramide, H2N−NO2; and a formal dimer of azanone, HNO. It is a relatively unstable compound that contains nitrogen, hydrogen, and oxygen atoms. The compound is not commonly encountered in pure form due to its instability and tendency to decompose.
Hyponitrous acid forms two series of salts, the hyponitrites containing the [ON=NO]2− anion, and the “acid hyponitrites” containing the [HON=NO]− anion.
Properties
- Chemical formula: H2N2O2
- Molar mass: 62.0282 g/mol
- Appearance: white crystals
- Conjugate base: Hyponitrite
Structure
The structure of hyponitrous acid involves two nitrogen atoms (N) and two oxygen atoms (O) connected by single bonds, with hydrogen atoms (H) bonded to the nitrogen atoms. The chemical formula indicates that there are two hydrogen atoms and two nitrous groups (containing one nitrogen and one oxygen atom each) in the molecule.
There are two possible structures of hyponitrous acid, trans and cis. trans-Hyponitrous acid forms white crystals that are explosive when dry. In aqueous solution, it is a weak acid (pKa1 = 7.21, pKa2 = 11.54), and decomposes to nitrous oxide and water with a half life of 16 days at 25 °C at pH 1–3:
H2N2O2 ⟶ H2O + N2O
H2N2O2 ⟶ H2O + N2O
Since this reaction is not reversible, N2O should not be considered as the anhydride of H2N2O2.
The cis acid is not known, but its sodium salt can be obtained.
Preparation
Hyponitrous acid (trans) can be prepared from silver(I) hyponitrite and anhydrous HCl in ether:
Ag2N2O2 + 2HCl ⟶ H2N2O2 + 2 AgCl
Ag2N2O2 + 2 HCl ⟶ H2N2O2 + 2 AgCl
Spectroscopic data indicate a trans configuration for the resulting acid.
It can also be synthesized from hydroxylamine and nitrous acid:
NH2OH+HNO2 ⟶ H2N2O2 + H2O
NH2OH + HNO2 ⟶ H2N2O2 + H2O
Reactivity
Hyponitrous acid is highly reactive and decomposes readily into nitric oxide (NO) and nitrous oxide (N2O) gases. This decomposition reaction is as follows:
2H2N2O2 → 2NO + N2O + 2H2O
Because of its instability and reactivity, hyponitrous acid is not typically isolated or used in practical applications. It is primarily of interest in the field of theoretical chemistry and as an intermediate compound in certain chemical reactions.