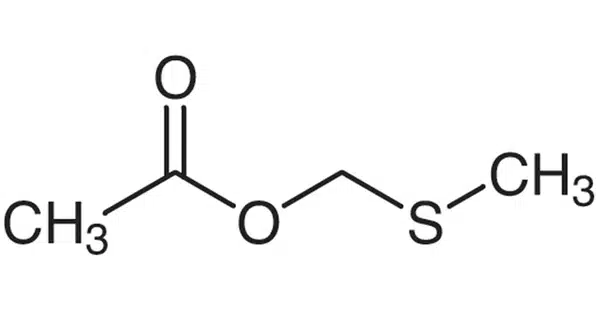
Methylthiomethyl is a three-part chemical molecule composed of a methyl group (CH3), a thio group (sulfur atom, S), and another methyl group. A methylthiomethyl (MTM) ether is a protecting group for hydroxyl groups in organic chemistry. Many chemical compounds contain hydroxyl groups, which must be protected during oxidation, acylation, halogenation, dehydration, and other reactions to which they are sensitive.
It has the chemical formula CH3SCH3 and is also known as dimethyl sulfide. This molecule contains a sulfur atom and is noted for its peculiar odor, which is often characterized as sweet, skunky, or cabbage-like.
Properties
- Molecular Weight: 90.19 g/mol
- Structure: MTM has a linear molecular structure with a sulfur atom bonded to a methyl group on either end.
- Physical State: Methylthiomethyl is typically a colorless, volatile liquid at room temperature.
- Odor: It may have a characteristic odor, but the smell can vary depending on impurities or other compounds present.
- Solubility: It is soluble in a wide range of organic solvents and is immiscible with water.
- Density: The density of MTM is greater than that of water.
- Boiling Point: The boiling point is around 88-89°C (190-192°F).
- Melting Point: The melting point is approximately -120°C (-184°F).
Many different types of protective groups for hydroxyl groups have been developed and employed in organic chemistry, but the number of protective groups for tertiary hydroxyl groups, which are susceptible to acid-catalyzed dehydration, is still limited due to their low reactiveness. They are easily protected with MTM ethers and recoverable with a high yield.
Methylthiomethyl is found in a variety of natural sources, including plants, vegetables, and marine species, and it is also linked to some industrial processes and environmental pollution.
There are two major techniques for introducing an MTM ether to a hydroxyl group. The first is a conventional Williamson ether synthesis in which an MTM halide is used as an MTM resource and sodium hydride (NaH) is used as a base. The other is a unique approach that employs dimethyl sulfoxide (DMSO) and acetic anhydride (Ac2O). In this scenario, the reaction happens via Pummerer rearrangement:
MTM ethers offer an additional benefit. Most other ethers are stable to neutral (but hazardous) mercuric chloride, which is used to eliminate them. As a result, selective deprotection of polyfunctional compounds using MTM ethers as protective groups for their hydroxyl groups is now achievable.
Applications
Methylthiomethyl is used as a reagent in organic synthesis, often as a source of a thiomethyl group in chemical reactions. It can be used in the modification of organic molecules to introduce sulfur-containing functional groups.
Safety
As with any chemical compound, proper safety precautions should be taken when handling MTM, including wearing appropriate protective equipment and working in a well-ventilated area.