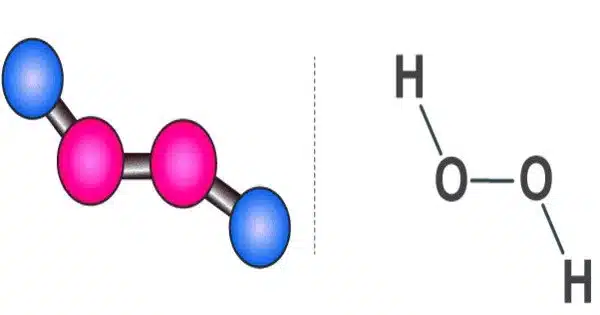
Hydrogen peroxide is a chemical compound with the formula H2O2. It is a pale blue liquid that is slightly more viscous than water. It is a very pale blue liquid that is slightly more viscous than water when pure. It is used as an oxidizer, bleaching agent, and antiseptic, usually in a dilute solution (3%-6% by weight) in water for consumer use, and in higher concentrations for industrial use. When heated, concentrated hydrogen peroxide, also known as “high-test peroxide,” decomposes explosively and has been used in rocketry as both a monopropellant and an oxidizer.
Hydrogen peroxide is a reactive oxygen species and the most basic peroxide, a compound with a single oxygen-oxygen bond. When exposed to light, it decomposes slowly into water and elemental oxygen, but quickly in the presence of organic or reactive compounds. It is typically stored in a dark bottle with a stabiliser in a weakly acidic solution to block light. The biological system, including the human body, contains hydrogen peroxide. Peroxidases are enzymes that use or decompose hydrogen peroxide.
Properties
The boiling point of H2O2 has been extrapolated as being 150.2 °C (302.4 °F), approximately 50 °C (90 °F) higher than water. In practice, hydrogen peroxide will undergo potentially explosive thermal decomposition if heated to this temperature. It may be safely distilled at lower temperatures under reduced pressure.
- Chemical formula: H2O2
- Molar mass: 34.0147 g/mol
- Appearance: Very light blue liquid
- Odor: slightly sharp
- Density: 1.11 g/cm3 (20 °C, 30% (w/w) solution); 1.450 g/cm3 (20 °C, pure)
- Melting point: −0.43 °C (31.23 °F; 272.72 K)
- Boiling point: 150.2 °C (302.4 °F; 423.3 K) (decomposes)
- Solubility in water: Miscible
- Solubility: soluble in ether, alcohol; insoluble in petroleum ether
Uses
- Hydrogen peroxide finds numerous applications in various fields, including:
- Disinfectant and Antiseptic: It is commonly used as a mild antiseptic and disinfectant to clean wounds, cuts, and scratches. It helps to kill bacteria, viruses, and fungi on the skin’s surface.
- Bleaching Agent: Due to its oxidizing properties, it is used as a bleaching agent for hair, textiles, paper, and other materials. It is often found in hair dyes, laundry detergents, and industrial processes.
- Water Treatment: It can be employed in water treatment systems to remove impurities and neutralize contaminants, including organic compounds and microorganisms.
- Rocket Propellant: In high concentrations, it can be used as a rocket propellant due to its decomposition into water and oxygen gas. This reaction releases a significant amount of heat and gas, creating thrust.
Safety Considerations
While hydrogen peroxide has many uses, it should be handled with caution. Higher concentrations can be corrosive, irritate the skin, and be toxic if ingested or inhaled. When using hydrogen peroxide, precautions should be taken, such as wearing gloves and protective clothing and adhering to proper handling and storage guidelines.